Preclinical & Pharmacy
The ABX-CRO Preclinical and Pharmacy department provides scientific consultancy for study design, coordination and conduct of proof-of-principle for radioactive and non-radioactive Investigational Medicinal Products/Investigational New Drugs (IMPs/INDs), at any stage of development. Translational studies tailored to the needs and requirements of a novel pharmaceutical agent can provide guidance and strong argumentation supporting further human clinical studies. We have an established network of qualified commercial research centres and academic institutes supporting all stages of development.
IMP/IND GMP Production Development
We support the development of radiolabelling of pharmacophores, sourcing and designing of linkers and biologicals, implementation of scalable GMP-production, or establishing of global IMP logistics solutions for your late-stage study
Preclinical Proof of Concept Studies
We design and implement complex pre-clinical studies to demonstrate the translational validity of novel pharmaceuticals as imaging or therapeutic agents. We utilise in vitro, ex vivo and in vivo assays using the appropriate models. In cooperation with our certified global imaging partner institutions, including an established network of qualified commercial research centres and academic institutes, we have access to preclinical services such as:
- In vitro assessment of novel compounds, e.g., affinity/specificity, kinetics, intracellular localisation and toxicity
- Animal studies including ex vivo biodistribution, pharmacokinetics, pharmacodynamics, toxicology, dosimetry and therapeutic efficacy, with access to a vast variety of suitable animal models, e.g., tumour rodent models; syngeneic, xenografts or patient-derived xenografts (PDX) induced subcutaneously or orthotopically, as well as non-human primates (NHP, i.e., marmosets, cynomolgus monkeys)
Toxicology studies
Toxicological requirements for biologicals, oncological drugs or radiopharmaceuticals are described in extensive ICH guidelines and are the last stage before FIH studies. These guidelines are often contradictory in real life when for example an established biological is radiolabelled and developed as a diagnostic or therapeutic agent for cancer. Our expert toxicologists will help you interpret the applicable guidelines, and design and implement a cost-effective preclinical package.
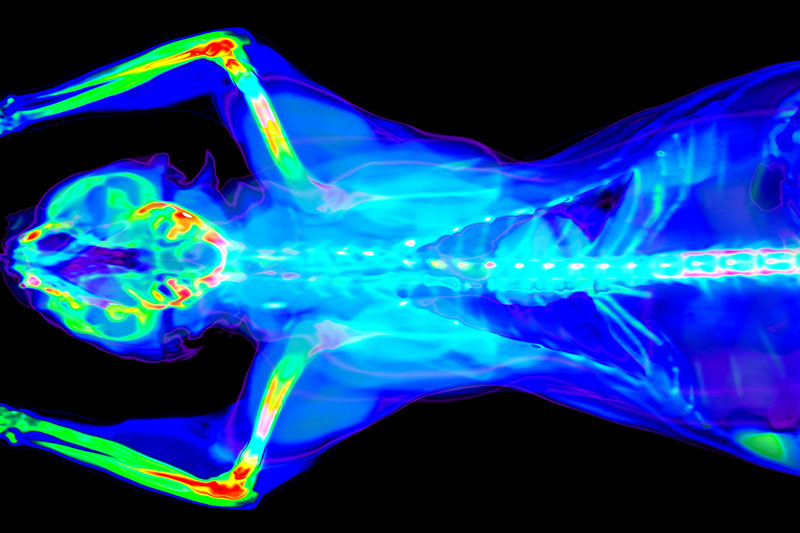
Imaging Studies
Imaging studies employing Molecular Imaging (SPECT, PET), Optical Imaging, CT, MRI or their combination, are of extreme value for drug development in indications such as oncology, cardiology, infectious diseases, inflammations and neurology. We offer Imaging-based projects in collaboration with leading institutions in Europe, North America and Japan, including:
- Imaging studies in model organisms of all sizes, from tumour-bearing rodents to minipigs
- CNS imaging in small or large non-human primates (marmoset, cynomolgus monkey)
- Advanced experimental or surgical interventions, performed by specially trained veterinarians
- Proof of concept dosimetry studies extrapolating data to human dose
- Imaging acquisition parameters set up and quantitative imaging data analysis, using standard or special solutions, optimised to suit study purposes.